Cancers, Vol. 12, Pages 3332: The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment
Cancers doi: 10.3390/cancers12113332
Authors: Huang Sun Sun Li Zhao Zhong Peng
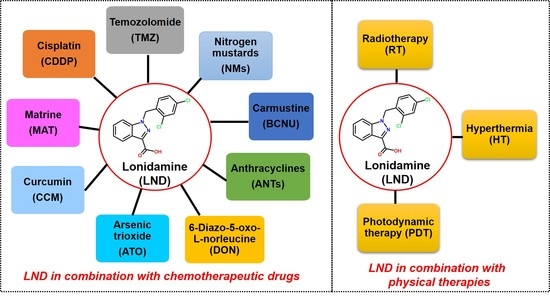
Lonidamine (LND) has the ability to resist spermatogenesis and was first used as an anti-spermatogenic agent. Later, it was found that LND has a degree of anticancer activity. Currently, LND is known to target energy metabolism, mainly involving the inhibition of monocarboxylate transporter (MCT), mitochondrial pyruvate carrier (MPC), respiratory chain complex I/II, mitochondrial permeability transition (PT) pore, and hexokinase II (HK-II). However, phase II clinical studies showed that LND alone had a weak therapeutic effect, and the effect was short and reversible. Interestingly, LND does not have the common side effects of traditional chemotherapeutic drugs, such as alopecia and myelosuppression. In addition, LND has selective activity toward various tumors, and its toxic and side effects do not overlap when combined with other chemotherapeutic drugs. Therefore, LND is commonly used as a chemosensitizer to enhance the antitumor effects of chemotherapeutic drugs based on its di sruption of energy metabolism relating to chemo- or radioresistance. In this review, we summarized the combination treatments of LND with several typical chemotherapeutic drugs and several common physical therapies, such as radiotherapy (RT), hyperthermia (HT), and photodynamic therapy (PDT), and discussed the underlying mechanisms of action. Meanwhile, the development of novel formulations of LND in recent years and the research progress of LND derivative adjudin (ADD) as an anticancer drug were also discussed.